Canada’s regulatory regime is criticized for preventing the adaptation of innovative technologies. The Conference Board of Canada found in 2021 that Canada ranked 11th among 16 countries and it was provided a “C” grade for extracting social and economic benefits from innovation. Adding to these observations, the 2021 OECD economic survey gave Canada a “poor” ranking, placing it among the bottom five countries, for Product Market Regulation partially due to persistent administrative burdens facing start-ups.
The federal government recently admitted that Canada has a slow one-size-fits all regulatory process which struggles to keep up with the speed of health and biosciences discoveries. There has been some effort to address this ongoing concern. Relieving the regulatory burden was a goal within Budget 2018 which allocated $11.5 million over three years to study how to modernize regulatory flexibility to reduce policy barriers. Industry stakeholders reported to the federal government some potential steps needed to modernise the regulatory process. The result was to increase efforts to become more agile and applicable to emerging health technologies such as big data, AI, gene editing and 3D printing to name a few.
Health Canada also identified that applying novel approaches to the regulatory environment would modernise the clinical trials framework to allow for the advancement of health technologies and sciences. In 2019, the government introduced what they called the “tailored authorization pathways for advanced therapeutic products”. The Advanced Therapeutics Products (ATPs) approval pathway, or ‘regulatory sandbox’, was created when sections 21.9 to 21.96 were added to the Food and Drug Act.
Regulatory Sandboxes Overview
Regulatory sandboxes emerged from the national regulatory barriers facing emerging financial technology (fintech) companies. The promise is assist novel business models with transforming the regulatory environment instead of trying to retrofit existing regulations. The United Kingdom’s Financial Conduct Authority (FCA) is credited with launching the first regulatory sandbox in 2016 defining it as: “a ‘safe space’ in which businesses can test innovative products, services, business models and delivery mechanisms without immediately incurring all the normal regulatory consequences of engaging in the activity in question”. The FCA anticipated this tool could reduce time to market by testing products while offering consumer protection safeguards, but not all regulators agree.
The Bank of Canada reported in 2017 that implementing sandboxes may provide regulatory advantage to emerging fintech companies over the established market participants. Similarly in 2018, the U.S. Securities and Exchange Commission’s (SEC) sentiments were less positive as the Commissioner stated: “The SEC’s role is not to hand out permission slips for innovation. Innovation happens—organically through private decisions and irrepressible human creativity.” Regardless the response from these central banks, there has been a rapid global uptake of sandboxes as the World Bank estimated in November 2020 that a total of 73 were operating among 57 jurisdictions.
Sandboxes in Canada
Presently, the only financial regulator to adopt this tool has been the Canadian Securities Administrators (CSA) focusing on crypto-assets. The Alberta government recently introduced the Financial Innovation Act (Bill 13) which is the first legislated approach to implement sandboxes across Acts that address finance, credit unions, consumer protection and other relevant Acts. Instead of a legislative route, the federal government may use regulatory powers to introduce the sandboxes.
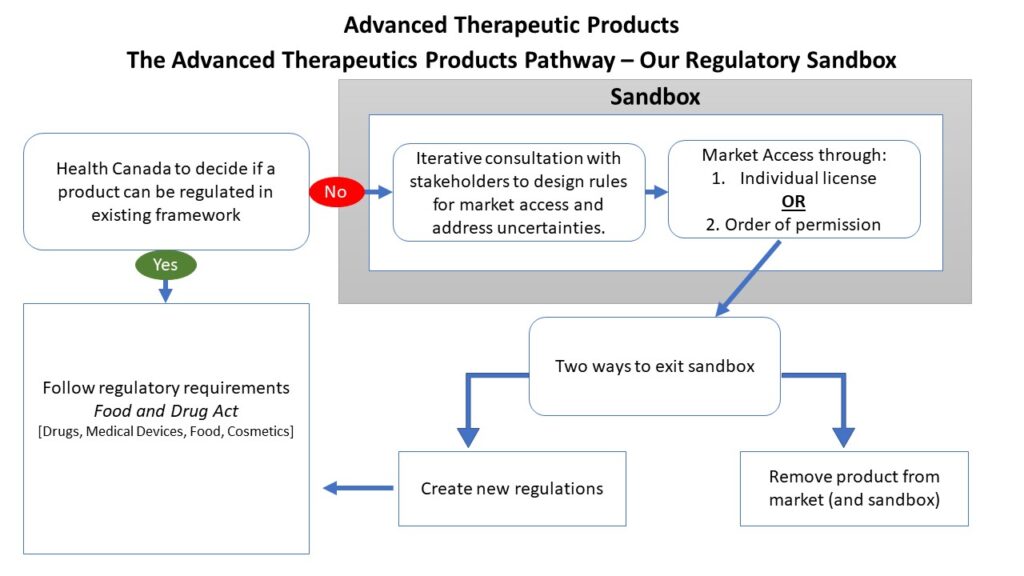
The Department of Justice Canada outlined how these tools will be implemented during a October 2020 presentation. The policy sectors where they will be applied include the health and biosciences, agriculture and agri-foods, transportation and infrastructure. The presentation also conceded that Health Canada’s product lifecycle was currently too slow potentially stifling the nation’s innovation and global competitiveness. The proposed pathway for the ATP regulatory sandbox is reproduced in Figure 1.
Figure 1: A reproduction of the ATP pathways on slide 25 from the Department of Justice Canada October 2020 presentation
Strategic Thoughts
Regulatory barriers stifle innovation and if left unchanged decrease public safety and Canada’s global competitiveness. The federal government is taking modest steps to modernise their regulatory process to accommodate innovative health and bioscience technologies. The ATP regulatory sandbox is still an isolated project-by-project approach, and it is difficult to know if it will have any system-wide impact.
Business and organizations operating within the health and biosciences space will benefit from a better understanding of how the federal and provincial governments are proposing to apply regulatory sandboxes to their industry. Early stage engagement will be essential to ensure these tools are structured to achieve their overall goal of reducing barriers to adapting socially beneficial technologies. Choosing to dismiss the opportunity to engage with the ATP will prevent innovators from participating in the direction of future regulations.
Ramsgate Policy Group monitors the pulse on Canada’s health care policy. Reach out to discuss how our strategic engagement can support your projects.
